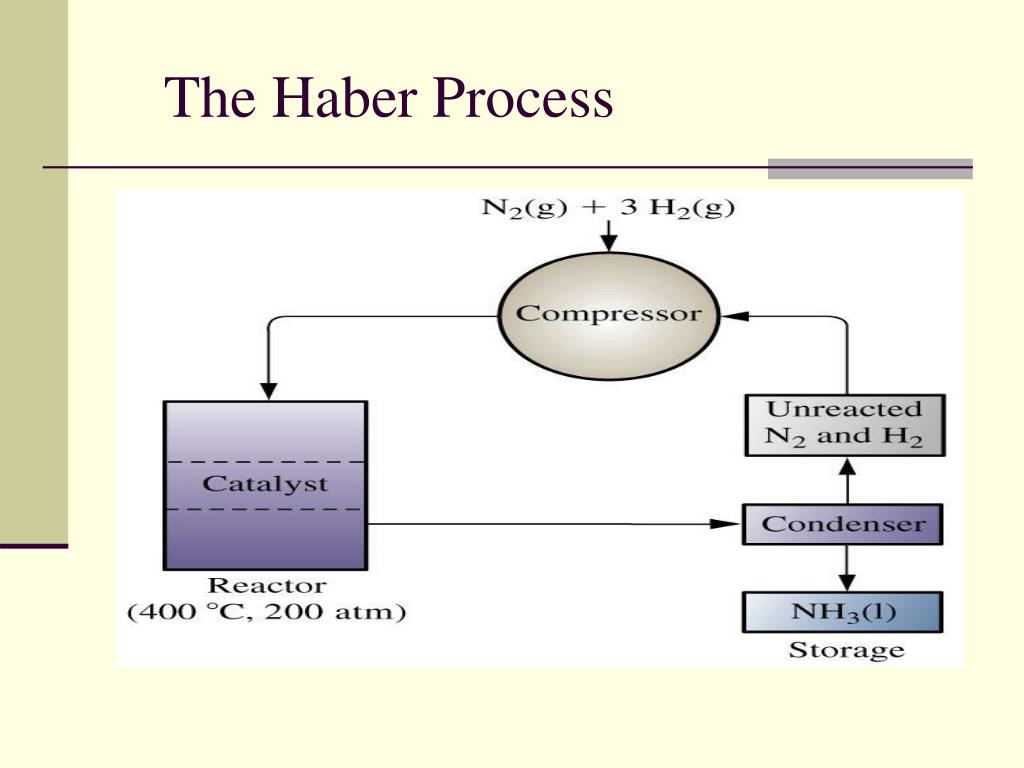
What Iron Catalyst Is Used In The Haber Process . The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. Transition metals are almost always used to catalyze the haber process. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. Despite all this, it has not so far been possible to determine experimentally. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia.
from www.slideserve.com
Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Transition metals are almost always used to catalyze the haber process. Despite all this, it has not so far been possible to determine experimentally.
PPT The Haber Process PowerPoint Presentation, free download ID6194678
What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Transition metals are almost always used to catalyze the haber process. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Despite all this, it has not so far been possible to determine experimentally. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations.
From onlineeducationnotesforeveryone.blogspot.com
HaberBosch Process What Iron Catalyst Is Used In The Haber Process Despite all this, it has not so far been possible to determine experimentally. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. Transition metals. What Iron Catalyst Is Used In The Haber Process.
From haber-boschprocess.blogspot.com
How does the Haber process feed the world? What is the HaberBosch What Iron Catalyst Is Used In The Haber Process Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Transition metals are almost always used to catalyze the haber process. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT The HaberBosch Process PowerPoint Presentation, free download What Iron Catalyst Is Used In The Haber Process Transition metals are almost always used to catalyze the haber process. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. Most often, industrial chemists use magnetite, an iron oxide (fe. What Iron Catalyst Is Used In The Haber Process.
From www.scienceabc.com
Haber Bosch Process Definition, Equation & Environmental Effects What Iron Catalyst Is Used In The Haber Process The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. Despite all this, it has not so far been possible to determine experimentally. Most often, industrial chemists use magnetite, an iron. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID6194678 What Iron Catalyst Is Used In The Haber Process Despite all this, it has not so far been possible to determine experimentally. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. A catalyst made mostly from iron enables the. What Iron Catalyst Is Used In The Haber Process.
From www.miragenews.com
LowTemp Ammonia Synthesis Breaks Barrier with Iron Catalysts and What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID3888209 What Iron Catalyst Is Used In The Haber Process The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Transition metals are almost always used to catalyze the haber process. Despite all this, it has not so far been possible. What Iron Catalyst Is Used In The Haber Process.
From spmchemistry.blog.onlinetuition.com.my
Haber Process SPM Chemistry What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. A catalyst made mostly from. What Iron Catalyst Is Used In The Haber Process.
From studymind.co.uk
Haber Process (GCSE Chemistry) Study Mind What Iron Catalyst Is Used In The Haber Process Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Transition metals are almost always used to catalyze the haber process. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. A catalyst made mostly from iron enables the reaction to be carried out at a. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT MATTER PowerPoint Presentation, free download ID6098351 What Iron Catalyst Is Used In The Haber Process Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Transition metals are almost always used to catalyze the haber process. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas. What Iron Catalyst Is Used In The Haber Process.
From slideplayer.com
The Haber Process. ppt download What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. A catalyst made mostly from. What Iron Catalyst Is Used In The Haber Process.
From vpics.homes
The Haber Process Amonia What Iron Catalyst Is Used In The Haber Process The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Despite all this, it has not so far been possible to determine experimentally. A catalyst made mostly from iron enables the reaction to be carried out. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID6194678 What Iron Catalyst Is Used In The Haber Process Transition metals are almost always used to catalyze the haber process. Despite all this, it has not so far been possible to determine experimentally. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and. What Iron Catalyst Is Used In The Haber Process.
From www.youtube.com
Iron as catalyst in Haber process Transition elements Sajjad What Iron Catalyst Is Used In The Haber Process Despite all this, it has not so far been possible to determine experimentally. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. Most often,. What Iron Catalyst Is Used In The Haber Process.
From gateways19.blogspot.com
The HaberBosch Process What Iron Catalyst Is Used In The Haber Process The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. A catalyst made mostly from iron enables the. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID3888209 What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. A catalyst made mostly from iron enables the reaction to be carried out at a lower temperature than would otherwise be. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT Equilibrium &The Haber Process PowerPoint Presentation, free What Iron Catalyst Is Used In The Haber Process The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations. Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. The haber. What Iron Catalyst Is Used In The Haber Process.
From www.slideserve.com
PPT Making ammonia The Haber process PowerPoint Presentation, free What Iron Catalyst Is Used In The Haber Process Most often, industrial chemists use magnetite, an iron oxide (fe 3 o 4. Despite all this, it has not so far been possible to determine experimentally. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the. Transition metals are almost always used to catalyze the haber. What Iron Catalyst Is Used In The Haber Process.